Distal Gastrectomy (Open)
, Massachusetts General Hospital
Main Text
Table of Contents
A complete margin-negative (R0) resection remains the only potentially curative treatment for gastric adenocarcinoma. The choice of operation depends on the location of the tumor as well as the stage of disease. This patient presented with symptomatic anemia, and workup demonstrated gastritis and a small tumor in the distal stomach. Biopsies confirmed adenocarcinoma, and an endoscopic ultrasound (EUS) staged this tumor as T2 N0. Staging scans showed no evidence of distant metastatic disease. Given that this patient had a relatively early stage tumor, we elected to proceed with upfront surgery, which in this case entailed a distal gastrectomy. This video shows an experienced gastric surgeon’s technique for performing an open distal gastrectomy with an “extended” D1 lymph node dissection.
Although the incidence of gastric cancer in the United States has dramatically decreased during the past several decades, gastric cancer remains an important cause of cancer-related death. Despite significant improvements in staging modalities, surgical therapy, and perioperative care, the prognosis of most patients with gastric cancer remains poor. This is largely a result of the aggressive biology of this cancer as well as the advanced stage of disease at which most patients present to the clinician. Risk factors include Helicobacter pylori and Epstein-Barr virus (EBV) infection, pernicious anemia, prior gastric resection, smoking, and high salt intake. Approximately 10% of gastric cancers are due to inherited cancer syndromes, including hereditary diffuse gastric cancer (HDGC), characterized by germline mutations in the E-cadherin (CDH1) gene, and hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, characterized by germline mutations in DNA mismatch repair genes. Recently, a comprehensive molecular evaluation of gastric cancer has led to a classification scheme that defines 4 major genomic subtypes and their approximate frequencies: EBV-infected tumors (9%), microsatellite unstable (MSI-high) tumors (22%), genomically stable tumors (20%), and chromosomally unstable tumors (50%).1
There are few, if any, classic symptoms suggestive of a diagnosis of stomach cancer, which explains why this cancer is frequently diagnosed at an advanced stage. Early symptoms, such as epigastric pain, dyspepsia, and acid reflux, are so nonspecific that most patients are simply treated empirically with antacids without further investigation. As the disease progresses, the symptoms become more prominent and more ominous and include such complaints as dysphagia, nausea, vomiting, early satiety, anorexia, fatigue, and weight loss. The location and histologic type of gastric cancer often dictates the symptom complex, from dysphagia for proximal tumors to vomiting for obstructing distal tumors to early satiety and weight loss for diffuse-type (linitis plastica) tumors.2,3
This patient is an 84-year-old gentleman with a history of gastritis and a chronic gastroesophageal junction stricture who presented to his primary care physician with symptoms of fatigue and shortness of breath. A complete blood count was obtained and showed that the patient was anemic. He denied abdominal pain, nausea, vomiting, change in bowel habits, difficulty eating, or weight loss.
The physical examination of a patient with gastric cancer is typically unremarkable with perhaps the exception of epigastric abdominal tenderness and, less likely, of a palpable epigastric mass. Classic eponymous physical findings such as Virchow’s node, Sister Mary Joseph’s periumbilical node, and Blumer’s shelf are rarely present but when seen indicate advanced metastatic disease. Similarly, the presence of cachexia, jaundice, ascites, and hepatomegaly in a gastric cancer patient typically signifies incurable metastatic disease. Our patient was well-appearing, and on examination he had a soft abdomen and no palpable masses.
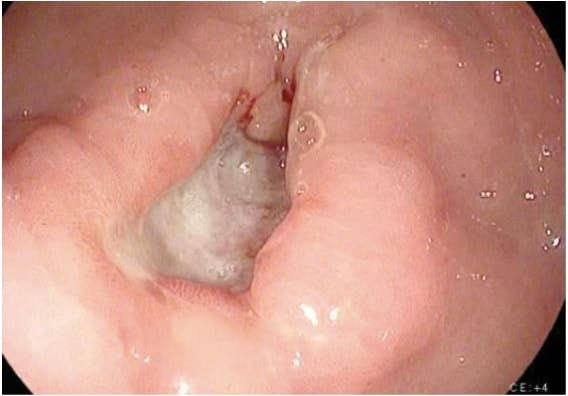
Imaging for workup of gastric cancer includes a variety of studies. The diagnosis of gastric cancer is usually readily established by endoscopy with biopsy (Fig. 1). Endoscopy defines the location and extent of the tumor within the stomach and, when combined with endoscopic ultrasound (EUS), allows for accurate estimation of the depth of tumor invasion (T stage). EUS is the most sensitive modality in establishing the T stage of a gastric cancer, and EUS enables an assessment of the regional lymph nodes and needle biopsy of suspicious nodes to confirm nodal involvement (N stage). A CT scan of the chest, abdomen, and pelvis should be performed to evaluate for distant metastatic spread, such as to the lungs, liver, peritoneum, or lymph nodes outside the field of resection. In addition, a CT scan is sensitive in detecting even small amounts of ascites that may represent peritoneal tumor spread and that can be sampled by image-guided means for cytologic examination. Though PET and PET–CT scans are not routinely recommended in the preoperative staging of gastric cancer, they may certainly provide additional useful information. PET has a low sensitivity in detecting the primary tumor, especially in early and diffuse-type gastric cancers, but PET has a higher specificity than CT (˜90% vs ˜60%) in the detection of regional lymph node metastases and has a reasonable sensitivity for the detection of liver, lung, and distant lymph node metastases.4 In addition, PET can be used to monitor tumor response to neoadjuvant chemotherapy, as it accurately detects responders to therapy at an early stage, thus enabling the clinician to maintain patients on as active a chemotherapy regimen as possible.
Patients with locoregionally advanced cancers (T3+/N+) are considered for additional staging by laparoscopy. Staging laparoscopy can be done immediately prior to the planned laparotomy or as a pretreatment procedure performed in patients considering preoperative therapy. Staging laparoscopy upstages more than 30% of patients through the identification of radiographically occult peritoneal and liver metastases and positive cytology.5
Gastric cancer typically spreads via the lymph nodes or hematogenously and commonly metastasizes to the liver, lungs, or peritoneum. It can also spread directly to involve adjacent organs, such as the pancreas and transverse colon. The only potential curative therapy for gastric cancer is surgery. Even with complete, margin-negative resection and (neo)adjuvant therapy, the 5-year survival rate remains low at ~40%.
The most common treatment paradigm for gastric cancer in the United States has historically been upfront surgery, followed by adjuvant chemoradiation therapy (as described in the Intergroup 0116 trial) for those patients with high-risk (T3/4, node positive, poorly cohesive-type) tumors.6 However, only 64% of the patients in this trial could receive the postoperative chemoradiotherapy as planned, and so many favor perioperative chemotherapy for such patients, as described in the MAGIC trial conducted by the British Medical Research Council. In this trial, patients were randomized to receive either perioperative chemotherapy (three cycles of epirubicin, cisplatin, and 5-fluorouracil (ECF) preoperatively and postoperatively) and surgery or surgery alone. Five-year survival rates were superior in the perioperative chemotherapy group compared with the surgery-alone group (36% vs. 23%).7 Though only ~ 40% of patients complete all of the recommended therapy with this treatment approach, more than 85% of patients receive all 3 planned cycles of preoperative chemotherapy, and all patients receive at least one cycle of chemotherapy. Administration of at least a few cycles of systemic therapy in advance of surgery for patients with high-risk tumors confers several advantages: (1) it permits early treatment of possible micrometastatic disease; (2) one can monitor the in vivo tumor response to the therapy, such as with PET–CT scan imaging; and (3) one can select for those patients with particularly bad tumor biology who develop early metastatic disease and who would thus derive no survival benefit from gastrectomy.7
A complete margin-negative (R0) resection remains the only potentially curative treatment for gastric adenocarcinoma. The choice of operation depends on the location of the tumor as well as the stage of disease. Superficially invasive (T1a) gastric cancers of favorable histologic grade can be treated by endoscopic mucosal resection or wedge excision with or without concomitant sentinel lymph node biopsy. Such procedures have been extensively described by our surgical colleagues from Japan, where gastric cancers are frequently diagnosed at an early stage given the prevalence of screening endoscopic examinations in that country. Because the majority of patients in the United States present with symptomatic, locoregionally advanced tumors, the primary surgical question is which procedure offers the greatest chance for cure with acceptable morbidity and mortality. Many patients are not candidates for any surgical procedure either because they are medically unfit or because of the presence of metastatic disease seen on preoperative imaging studies or at the time of laparoscopy. For those patients who are candidates for gastric resection, the options include total, proximal, and distal gastrectomy. There is an increasing worldwide experience in performing these procedures laparoscopically, with improved short-term outcomes and nodal yields, and survival outcomes that are on par with the traditional open procedures. However, the vast majority of resections for gastric cancer in the United States are still performed via the open approach.
Patients who should be considered for immediate surgical resection include those with early stage (T1/T2 N0) gastric cancers and those who require immediate palliation of bleeding or high-grade tumor-associated luminal obstruction. However, the perioperative morbidity and mortality rates in this latter population of patients are significant and must be carefully weighed against the likely benefits of resection. Patients with locoregionally advanced gastric cancers are good candidates for preoperative chemotherapy or chemoradiation therapy.
Because this patient had a relatively early stage tumor (uT2N0) and was in remarkably good health for his age, he was offered upfront surgery with a distal gastrectomy. We felt that an open distal gastrectomy limited the risk of overtreatment with neoadjuvant chemotherapy for a potentially low-risk cancer, while leaving us the option to offer him adjuvant therapy if the final pathology report revealed a more advanced (eg, T3+/N+) cancer.
The extent of gastric resection is determined by the location and extent of the primary tumor. Prospective, randomized trials failed to demonstrate a survival advantage for total gastrectomy over distal, subtotal gastrectomy for patients with tumors of the distal stomach.8 Therefore, for those patients in whom a 5- to 6-cm margin from the tumor can be obtained while still maintaining a reasonably sized gastric remnant, a more conservative gastric resection should be performed, as this confers an equivalent survival rate with less morbidity and a better quality of life than does a total gastrectomy.8 Nonetheless, a total gastrectomy should be performed if necessary to achieve an R0 resection, as positive resection margins (R1 resections) lead to very poor survival. In the Dutch gastric cancer trial, 10% of patients had a positive resection margin and a correspondingly inferior 3-year survival (18% vs 63%) compared with those who had a negative resection margin.9 However, microscopically involved margins appear to affect long-term survival only in those patients with five or fewer lymph node metastases. For proximal gastric cancers, many surgeons prefer a total gastrectomy over a proximal gastrectomy owing to the long-term sequelae of symptomatic acid reflux with the latter procedure.
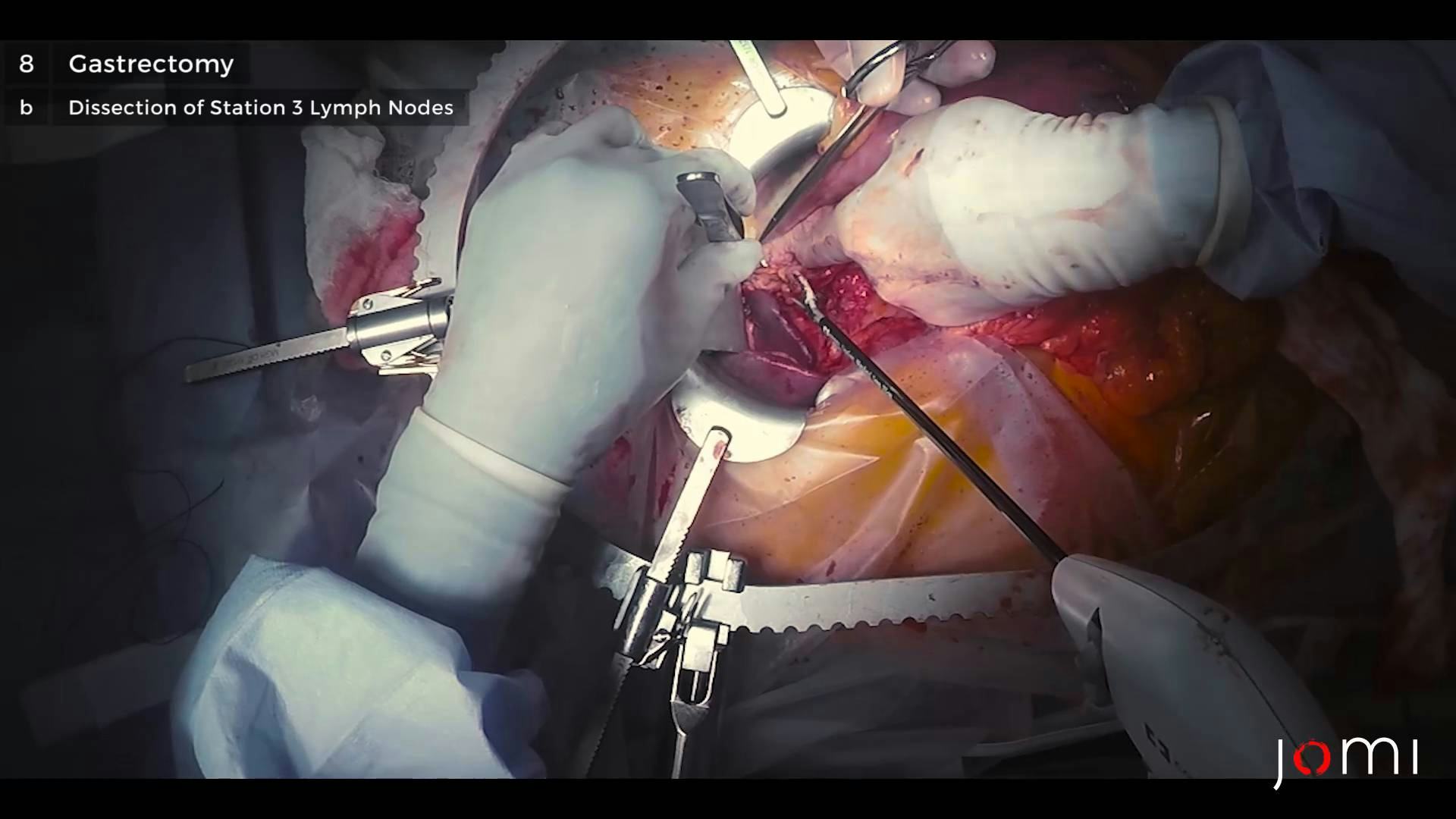
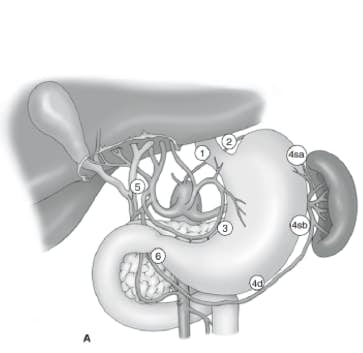
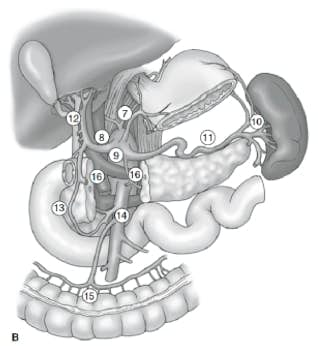
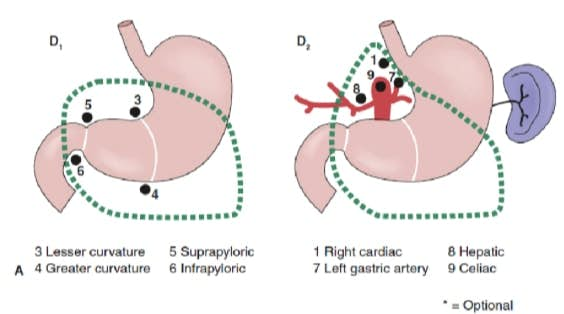
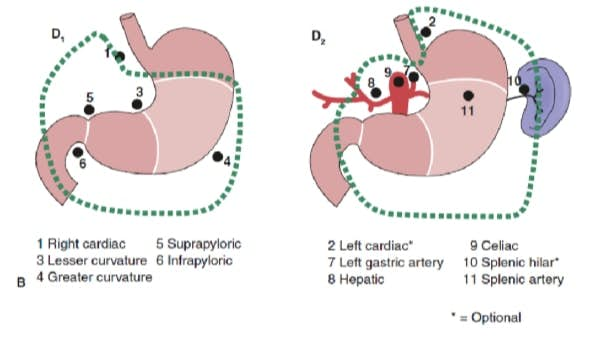
The extent of lymph node dissection is one of the most controversial issues in the management of gastric cancer. Reports from the Gastric Cancer Registry and other retrospective studies have made radical gastrectomy with extended (D2) lymphadenectomy the standard of care for the treatment of curable gastric cancer in Japan.10,11 The Japanese Research Society for Gastric Cancer categorizes the draining lymph node basins of the stomach into 16 stations, including 6 perigastric stations (Fig. 2a) and 10 regional stations along the major vessels and adjacent to the pancreas (Fig. 2b). The extent of lymph node dissection is indicated by the designation D. A D1 dissection includes only the perigastric nodes (stations 1 to 6); a D2 dissection includes the lymph nodes along the common hepatic, left gastric, celiac, and splenic arteries (stations 7 to 11); and a D3 dissection includes additional nodes within the porta hepatis and adjacent to the aorta (stations 12 to 16) (Fig. 3).
Two large, prospective randomized trials comparing the outcomes of D1 dissection with those of D2 dissection have been conducted in Western patients. Fifteen-year follow-up of the larger of these two studies, namely, the Dutch Gastric Cancer Group Trial, demonstrated no long-term overall survival benefit with the D2 lymph node dissection (29%% vs 21%, P = .34) but did show a significantly decreased gastric cancer-related death rate (37% vs 48%, P = .01) and a lower locoregional recurrence rate than D1 surgery.9 However, there were significantly higher rates of postoperative morbidity (43% vs 25%) and mortality (10% vs 4%) in those patients undergoing the more extensive D2 lymphadenectomy, in large part resulting from the greater numbers of splenectomy and pancreatectomy performed in this group in order to achieve a complete node dissection.9 Similarly, the British Cooperative trial conducted by the Medical Research Council reported an increased morbidity, associated with high rates of concomitant splenectomy and pancreatectomy, without a benefit in overall or recurrence-free survival in those patients randomized to a D2 dissection.12
Despite the findings of these two studies, investigators have argued that if the complication rate after an extended D2 lymph node dissection could be decreased, the survival benefit conferred by a D2 dissection, as reported by experienced centers in Asia, might be translated to Western patients with gastric cancer.13 Thus, the Italian Gastric Cancer Study Group conducted a phase III trial randomizing patients to either a pancreas-preserving D2 lymphadenectomy or a D1 lymphadenectomy at high-volume centers by surgeons who completed rigorous training in the technique through observation of master surgeons in Japan. They reported impressively low morbidity (17.9%) and mortality (3.0%) rates in patients undergoing the D2 dissection, in which a pancreatectomy or splenectomy was performed only for direct tumor invasion.14 However, they found no difference in 5-year overall survival between the D1 and D2 groups (66.5% vs 64.2%; P = 0.695), though subgroup analyses suggested that a D2 lymphadenectomy may be of benefit to patients with T2 – T4 and/or node-positive tumors.14
It remains unclear whether a D2 lymph node dissection is simply a thorough staging procedure or whether there may be a therapeutic benefit for certain subsets of patients (eg, patients with N2 disease). It should be noted that the accurate staging of patients with gastric cancer, according to the most recent seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual, demands the evaluation of at least 15 lymph nodes.15 The nodal staging is then based on the number of positive nodes with N1 (1 to 2 positive nodes), N2 (3 to 6 positive nodes), N3a (7 to 15 positive nodes), and N3b (16+ positive nodes) categories. Studies have shown a correlation between improved patient survival and larger numbers of lymph nodes examined in the specimen.16 Of course, this may simply reflect more accurate staging as opposed to a therapeutic benefit from the procedure. However, there is indirect evidence that more extensive lymphadenectomies result in lower rates of locoregional recurrence, and this may translate into a survival benefit. Indeed, a randomized trial conducted at a single center in Taiwan identified an overall 5-year survival advantage for those patients undergoing a D3 dissection (59.5%) compared with those undergoing a D1 dissection (53.6%).13
For patients treated at MGH over the past decade, we have reported morbidity and mortality rates after gastrectomy and D2 lymphadenectomy of 17% and 0%, respectively, and our median lymph node yield with ex vivo dissection of the specimen has increased to 40 nodes. The median length of stay for subtotal and total gastrectomy in the United States averages 9 – 10 days, though many patients are discharged within 7 days. The long-term complications of subtotal and total gastrectomy include vitamin and mineral deficiencies, particularly of vitamin B12, vitamin D, iron, and calcium, and the classic postgastrectomy syndromes, such as alkaline reflux gastritis, dumping syndrome, Roux stasis syndrome, and afferent limb syndrome.
Surgery is the only potentially curative therapy for localized gastric cancer, yet even for those patients in whom an R0 resection is possible, only 35% to 40% 5-year survival is achieved with either perioperative chemotherapy or postoperative chemoradiotherapy. Future progress in the treatment of gastric cancer depends on the development of better systemic therapies as well as techniques for earlier diagnosis.
A. DISTAL STOMACH RESECTION:
Gastric adenocarcinoma, tubular type, mismatch repair protein expression instable. (See synoptic report).
B. LYMPH NODE BIOPSY, STATION 8:
There is no evidence of malignancy in four lymph nodes (0/4).
C. LYMPH NODE BIOPSY, STATION 11:
There is no evidence of malignancy in two lymph nodes (0/2).
D. LYMPH NODE BIOPSY, STATION 7:
There is no evidence of malignancy in two lymph nodes (0/2).
E. LYMPH NODE BIOPSY, STATION 3:
There is no evidence of malignancy in one lymph node (0/1).
SYNOPTIC REPORT:
TUMOR STAGE SUMMARY: pT1bN0.
SPECIFIC SITE: Gastric antrum.
TUMOR SIZE (Greatest dimension): 0.3 cm (as measured on slide).
WHO CLASSIFICATION: Tubular adenocarcinoma.
HISTOLOGIC GRADE: G1 (Well differentiated)
EXTENT OF INVASION: pT1b (Tumor invades submucosa).
SMALL VESSEL (BLOOD/LYMPHATIC) INVASION: Absent.
LARGE VESSEL (VENOUS) INVASION: Absent.
PERINEURAL INVASION: Absent.
PROXIMAL GASTRIC MARGIN: Uninvolved by invasive carcinoma.
DISTAL DUODENAL MARGIN: Uninvolved by invasive carcinoma. (clearance= 0.4 cm).
REGIONAL LYMPH NODES: pN0 (No regional lymph node metastasis): 31 lymph nodes examined.
*The lymph node total is inclusive of all specimen parts. The main specimen (specimen A) contained 22 lymph nodes.
HER2 IMMUNOHISTOCHEMISTRY: Her 2 score 0/negative (No reactivity or very faint membranous staining in < 10% of tumor cells).
Additional Studies:
MLH1 and PMS lost.
MSH2 and MSH6 intact.
Additional findings:
Gastric antral mucosa with intestinal metaplasia and high grade dysplasia.
Fundic gland polyp.
Epstein-Barr virus encoded RNA (EBER) is negative.
Our patient recovered well from surgery and was discharged on postoperative day 5. His final pathology report staged his tumor as T1bN0, and the margins of resection were negative. We achieved an excellent nodal harvest (31 lymph nodes), so we can be confident in the accuracy of this stage assignment. As such, he does not require adjuvant therapy, and his long-term prognosis is excellent.
A fixed abdominal wall retractor system can be very helpful for this operation; we used the Bookwalter, but other options include the Thompson and Omni retractor systems. The duodenum and stomach are transected with staplers -- in this case an Endo-GIA stapler. A vessel sealing device, such as a LigaSure device or Harmonic scalpel, are quite helpful in the efficient control of small blood vessels. Larger vessels, such as the left gastric artery, can be divided with a vascular stapler or controlled by traditional suture ligation.
The authors have no conflicts to disclose.
The patient referred to in this video article has given his informed consent to be filmed and is aware that his personal health information will be published online in unidentified fashion.
Citations
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202-209. doi:10.1038/nature13480.
- Mullen JT. Gastric cancer. In: Fischer J, ed. Mastery of Surgery. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017.
- Yoon SS, Park DJ. Gastric adenocarcinoma. In: Cameron JL, Cameron AM, eds. Current Surgical Therapy. 11th ed. Philadelphia, PA: Saunders, 2014:87-95.
- Yun M. Imaging of gastric cancer metabolism using 18 F-FDG PET/CT. J Gastric Cancer. 2014;14(1):1-6. doi:10.5230/jgc.2014.14.1.1.
- Ikoma N, Blum M, Chiang YJ, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23(13):4332-4337. doi:10.1245/s10434-016-5409-7.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725-730. doi:10.1056/NEJMoa010187.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. doi:10.1056/NEJMoa055531.
- Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L; Italian Gastrointestinal Tumor Study Group. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Ann Surg. 1999;230(2):170-178. doi:10.1097/00000658-199908000-00006.
- Bonenkamp JJ, Hermans J, Sasako M, et al; Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340(12):908-914. doi:10.1056/NEJM199903253401202.
- Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric surgery in Japan and its limits of radicality. World J Surg. 1987;11(4):418-425. doi:10.1007/BF01655804.
- Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82(3):346-351. doi:10.1002/bjs.1800820321.
- Cuschieri A, Weeden S, Fielding J, et al; Surgical Co-operative Group. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer. 1999;79(9-10):1522-1530. doi:10.1038/sj.bjc.6690243..
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised control trial. Lancet Oncol. 2006;7(4):309-315. doi:10.1016/S1470-2045(06)70623-4.
- Degiuli M, Sasako M, Ponti A; Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97(5):643-649. doi:10.1002/bjs.6936.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23(28):7114-7124. doi:10.1200/JCO.2005.14.621.